pf3 molecular geometry|Geometry of Molecules : Clark A quick explanation of the molecular geometry of PF3 including a description of the PF3 bond angles. Looking at the PF3 Lewis structure we can see that .
What percentage of global greenhouse gas emissions does Timor-Leste produce? Timor-Leste produced 0.01% of global greenhouse gas emissions in 2021 (the latest date with complete emissions data). This amounted to 5.75m metric tonnes of carbon dioxide equivalent, or MtCO₂e. These emissions represented an increase from 2020 by .
PH0 · Phosphorus trifluoride
PH1 · PF3 lewis structure, Molecular geometry, Bond angle, Hybridization
PH2 · PF3 Molecular Geometry / Shape and Bond Angles
PH3 · PF3 Lewis Structure, Molecular Geometry, and Hybridization
PH4 · PF3 Electron Geometry (Phosphorus trifluoride)
PH5 · Lewis Structure of PF3 (With 6 Simple Steps to Draw!)
PH6 · Geometry of Molecules
PH7 · Chemistry Learning Made Easy: PF3: Lewis Structure and
PH8 · 8.6: Molecular Geometries
PH9 · 5.9: Molecular Geometry
Level up your lottery game with the OLG App. Buy tickets, set reminders, and check your winnings easily. Play LOTTO MAX, LOTTO 6/49, and more. Also, enjoy a variety of casino and instant games for your chance to win big!
pf3 molecular geometry*******Learn how to draw the Lewis structure, molecular geometry, and hybridization of phosphorus trifluoride (PF3), a tetra-atomic gas with a bent structure. Find out the bond angle, polarity, and lone pair effects of PF3 molecule. Tingnan ang higit paAs per this rule, the maximum number of valence electrons an atom can have is eight. One phosphorus atom has five valence electrons, having a scarcity of three to . Tingnan ang higit pa
The electrons present in the outermost shell of an atom are called valence electrons. Because they are present in the outermost shell, the hold of the nucleus is weak on . Tingnan ang higit paThe geometrical structure of the tetra-atomic Phosphorus Trifluoride (PF3) molecule is studied with the help of the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory explains that the bond angle between the fluorine-phosphorus . Tingnan ang higit paThe Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms . Tingnan ang higit pa A quick explanation of the molecular geometry of PF3 including a description of the PF3 bond angles. Looking at the PF3 Lewis structure we can see that . For the molecular geometry, looking at the PF3 Lewis structure we can see that there are three Fluorine (F) atoms attached to the central Phosphorus (P) atom and that there is one lone pair of. Why is the molecular geometry of PF3 is trigonal pyramid whereas its electron geometry is tetrahedral? This is because there is a slight difference in molecular .
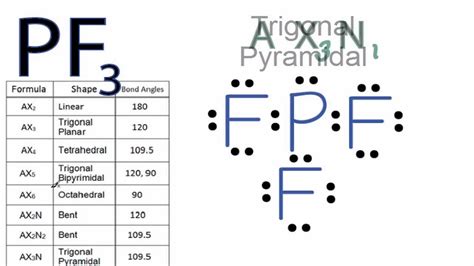
Learn how to draw the Lewis structure and determine the molecular geometry for phosphorus trifluoride (PF3) with this video by chem101csub. The video explains the steps and shows the results for PF3.There is a three step approach to determining the geometry of a molecule. Determine the Lewis dot structure of the compound. Determine the Electron geometry from the Lewis dot structure. Determine the molecular .Formula: F 3 P. Molecular weight: 87.968972. IUPAC Standard InChI:InChI=1S/F3P/c1-4 (2)3 Copy. IUPAC Standard InChIKey:WKFBZNUBXWCCHG-UHFFFAOYSA-N Copy. .Geometry of Molecules 6 Steps to Draw the Lewis Structure of PF3 Step #1: Calculate the total number of valence electrons. Here, the given molecule is PF3 (phosphorus trifluoride). In order to draw the lewis structure of . Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the .
Predicting Electron Pair Geometry and Molecular Structure. The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures: Write the Lewis . The VSEPR shape of the molecule "PF"_3 is trigonal pyrimidal. We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule PF_3 because of its AX_3E status. VESPR stands for . Phosphorus trifluoride (formula PF3), is a colourless and odourless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in meta. Molecular Geometry of BF3. Each molecule has its shape that can be represented after finding out their electrons hybridized in various types of s-orbital and p-orbital like sp2, sp3, sp. As you found .The molecular geometry of the PF_3 molecule is _____ and this molecule is _____. a) trigonal planar, polar b) trigonal planar, nonpolar c) trigonal pyramidal, polar d) trigonal pyramidal, nonpolar e) tetrahedral, unipolar

Quantity Value Units Method Reference Comment; Δ r H°: 64.0 ± 4.2: kJ/mol: IMRE: Larson, Szulejko, et al., 1988: gas phase; B,M Quantity Value Units Method .Question: What is the molecular geometry of PF3? Enter the molecular geometry of the molecule. Show transcribed image text. There are 2 steps to solve this one.
The electron-pair geometry and molecular structure are identical, and CO 2 molecules are linear. (b) We write the Lewis structure of BCl 3 as: Thus we see that BCl 3 contains three bonds, and there are no lone pairs of electrons on boron. The arrangement of three regions of high electron density gives a trigonal planar electron-pair geometry.
The molecular geometry of PF3 (Phosphorus trifluoride) is trigonal pyramidal and it is a polar molecule due to the electronegativity differences between phosphorus and fluorine atoms forming polar bonds. Explanation: The molecular geometry of the PF3 molecule is trigonal pyramidal. The PF3 molecule contains one phosphorus . This arrangement of the atoms makes the molecular geometry of PF 5 Trigonal Bipyramidal. PF5 Bond Angles. As mentioned earlier, the fluorine atoms in PF 5 either occupy the equatorial position or axial one; there are two bond angles for this molecule. The bond angles for the Fluorine atoms in the equatorial position, F-P-F is 120°.
Molecular Geometry: The PF3 molecule consists of one phosphorus atom (P) and three fluorine atoms (F). To determine the molecular geometry, we need to consider the arrangement of the atoms around the central phosphorus atom. The phosphorus atom has one lone pair of electrons and three bonding pairs of electrons.
Determine the electron geometry, molecular geometry, and idealized bond angles for each of the following molecules. In which cases do you expect deviations from the idealized bond angle? PF3. SBr2. CH4. COCl2
The molecular geometry of the PF 3 molecule is _____, and this molecule is _____. Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on. 7.6: Molecular Structure and Polarity VSEPR theory predicts the three-dimensional arrangement of atoms in a molecule. It states that valence electrons will assume an electron-pair geometry that minimizes repulsions between areas of high electron density (bonds and/or lone pairs).
This would make the electron geometry tetrahedral. However, this is not the molecular geometry. Two of these attachments are bonds and the other two are lone pairs. Therefore, the resulting molecular geometry is a a bent geometry. Now that we know the molecular geometry, we can determine the bond angle to be about 105 degrees from our chart.
Question: The molecular geometry of the PF3 molecule is and this molecule is A) tetrahedral, unipolar B) trigonal pyramidal, nonpolar C) trigonal planar, nonpolar D) trigonal pyramidal, polar E) trigonal planar, polar . Show transcribed image text. There are 2 .
We show you how to draw the Lewis structure and determine the moleculargeometry for phosphorus trifluoride (PF3).pf3 molecular geometry Geometry of Molecules Identify the electron-group geometry, molecular structure, and bond angles. Then try to find a chemical formula that would match the structure you have drawn. Answer. Answers will vary. For example, an atom with four single bonds, a double bond, and a lone pair has an octahedral electron-group geometry and a square pyramidal molecular structure.
pf3 molecular geometry Molecular Geometry of PF3. Answer: The molecular geometry of Phosphorus Trifluoride (PF3), a molecule consisting of one phosphorus atom and three fluorine atoms, is essential for understanding its chemical behavior, physical properties, and reactivity.Here’s a detailed look at the molecular geometry of PF3: 1. Lewis Structure. .
mega hd porn HD videos We found 194 videos to your request what happens in the break room., 4K 27m 54s. no fuckdoll like stepsis 39m 56s. raise it harder for this ass 27m 51s. will you study with emptied balls? 32m 28s. today is my chosen day for sex, bro 24m 55s. have a taste of something sweeter
pf3 molecular geometry|Geometry of Molecules